Understanding the Laboratory Proficiency Testing Cycle (External Quality
Assessment – EQA)
This presentation is part 3 of a series highlighting
Proficiency Testing as a Laboratory Quality Partner.
This series is put together
by Dr. Michael A Noble BA MD FRCPC, the material founder and now retired chair of
CMPT – Canadian (formerly Clinical) Microbiology Proficiency Testing Program. It
is based on his national and international experience and expertise in
Proficiency Testing (PT/EQA). Dr Noble continues as a member on the advisory committee of
CMPT and collaborates with other EQA programs including Oneworld Accuracy
In 1946 F. W. Sunderman returned to the United States after serving in the military
to find that community confidence in medical laboratories was so low that it became common practice
to send samples to multiple laboratories and mean results rather than trust the
results of any single laboratory.
He was so concerned that he set up the first
Quality Assessment study for common chemistry analysis in medical laboratories.
He learned, with concern, the community was right in their concern on laboratory
reliability
(see: Belk WP, Sunderman FW. Am J Clin Pathol. 1947
Nov;17(11):853-61).
Shortly after, the College of American Pathologists began
its national Proficiency Testing program. Much has changed in medical
laboratories over the intervening 75 years, but as long as laboratorians test
and report on samples, there is a continuing need for monitoring performance
using Proficiency Testing, also referred to as External Quality Assessment
(EQA) laboratories regardless of industry. It is specified in both ISO/IEC 17025:2017
(General requirements for the competence of testing and calibration
laboratories) and in ISO15189:2012* (Medical laboratories — Requirements for
quality and competence).
*Note: ISO Standards 15189 and 17043 are about to be republished as :2023 The new standards are not currently available
PT/EQA process of quality assessment is also a subject to
accreditation published as ISO/IEC17043:2010* (Conformity assessment — General
requirements for proficiency testing). I suspect that the process of assessment
has not fundamentally changed substantially since Sunderman’s first early works, but it is
now much better defined.
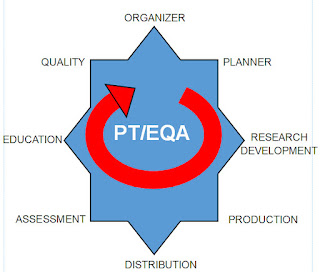
To better visualize the process I have created a cycle graphic
From my perspective PT/EQA must keep in mind the Eight Positions
of a performance cycle. I have named these positions by the key person or
persons holding that position, starting with the Organizer and ending with the Quality Team. The graphic indicates this is a cycle that repeats with each test challenge and event.
These positions do not work in isolation silos; they work in teams, depending on the task. I see four teams:
Team 1: The Quality Team
Team 2: The Preparation Team
Team 3: The Distribution Team
Team 4: The Assessment-Education Team, and then back to Team 1.
Depending on the size and complexity of the
organization, each position may represent a unique person or group. In a smaller
organization, especially if they are just getting started, they may well find
that number of people involved is fewer, although I doubt that any group would
be able to succeed with fewer than four. In the olden days, it was possible to
start with little specific knowledge about the process of EQA and learn as you
go. I doubt that is the case any longer.
PT/EQA cycle.
Team 1- The Quality Team. Organizer and Quality
The
Organizer
As with all things laboratory, the PT/EQA Cycle begins and ends with
Quality. (Indeed Quality is integral throughout). Among many activities, the
Organizer is the person responsible for setting the mission and vision and
quality policies of the program. The organizer sets the annual goals and
objectives, and reviews quality performance and has the sign-off responsibility for the program Quality Manual.
The Organizer is the one responsible for the program's Annual Report,
including the review of non-conformances and opportunities for improvement. They report on if the organization met its expected goal and objectives, and presents its expectations for coming year. The
Organizer is, in ISO terms, the lead of Top Management. If the Organizer is not
Quality engaged, the program will ultimately fail.
Quality
At the end
of every test event the Quality Team needs to review not only the results of
laboratory performance, but even more importantly, the performance of the EQA
scheme.
Did the samples meet the expectations of scheme? Did they meeting
homogeneity and stability requirements? Did the get sent at the right time and
did they go to the right places? Did they make clear the contents with respect
to instructions and safety? Was the expected date for reporting clear? Were the
results reviewed prior to sending out. Did the scheme receive any complaints,
concerns, or comments or compliments on the results? Were any-and-all addressed
as required? Did the results of this test event lead to a concern about any
suppliers, or about any staff members? Were there any opportunities for
improvement identified, and if so was an investigation pursued? Did this
initiate an internal audit, and if so was it completed and reviewed?
Does the
Quality Team and the Organizer, have their eye on their own Accreditation
status? Are they in-step with their own requirements? Are they ready to consider
whether or not they want, need or require to taking their first important step
towards their own Accreditation?
PT/EQA has a position of incredible power and
influence that can impact on laboratories reputation and accreditation. It holds
that position because of its close attention to its own quality status.
The next post will highlight
Team 2: Organizer- Planner- Research&Development- Production